For the last two decades, breast augmentation remains at the top of the chart of all plastic surgery procedures performed world wide. Breast Augmentation has evolved significantly since the time of its inception in terms of implants, technique and overall safety of the procedure. A better understanding of the surgical planning and surgical technique has resulted in improved results and more predictable outcomes.
The FDA has approved implants for increasing breast size in women, for reconstruction after breast cancer surgery or trauma, and to correct developmental defects.
Today we have three different options available for Breast Augmentation.
1.Breast Implants remain the gold standard procedure for Breast Augmentation as it provides definitive and predictable enhancement in the size of the breast.
- Autologous fat transfer: in this procedure one’s own body fat is harvested from areas where it is in excess and transferred to the breast in specific planes to increase breast volume. Since some amount of fat is resorbed by the body, the resulting volume augmentation in the breast is very modest. Hence patients more often require a repeat session to achieve desired augmentation.
- Composite Augmentation: it is a relatively new technique of Breast Augmentation
where breast implant and fat transfer are combined to enhance the breast volume.
Breast Augmentation in the 1980’s and 90’s largely focused on volume augmentation where the sole aim of the procedure was volume increase. Today we focus on techniques that help in better coverage of implants even in the skinniest breast. Composite Breast Augmentation allows us to add volume in specific areas of breast especially in upper part of the breast and allows possible cleavage enhancement. Composite breast augmentation is a great addition in our armamentarium especially in patients who have tuberous breast ( patients suffering from underdevelopment of breast with a constricting band of tissue within the breast).
Earlier breast augmentation approach was limited to subglandular or submuscular
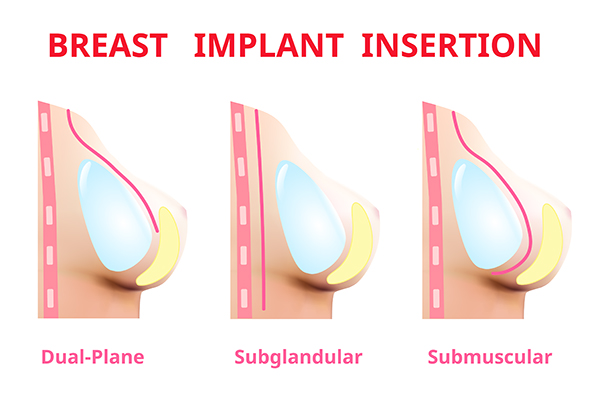
Fig : 1 Different planes of breast implant placement.
placement of implant. In present time we have options of placing implant in the subfacial plane and dual plane. We choose these approaches based on clinical evaluation, hence the procedure is customized to ones need. Therefore, we are now able to give predictable outcomes and improved results.
The FDA has approved two types of breast implants: saline-filled (salt water solution) and silicone gel-filled. Both have a silicone outer shell and vary in size, shell thickness, shell surface texture, and shape (contour).
Breast implant design and manufacturing process has continued to improve and evolve. Successive developments of breast implants are referred to as generations. The most current breast implant in the market are 5th generation device. These implants are characterized by high standards of manufacturing, they have a reliable barrier levels in the shells that makes them quite resilient to damage there by reducing the incidence of implant rupture or tear. The gel is form stable and they come in wide range of anatomic and round matrices. The implants come in a range of textures, from smooth to nano, micro and macrotexture as well as polyurethane. Perhaps the most recent innovation in breast implant design has heralded the era of 6th generation breast implant devices which are called as light weight implants- these are claimed to be 30 % lighter than silicone gel filled implants. It is composed of cohesive silicone gel bonded with lightweight microspheres there by making them lighter than the conventional silicone implant. Light weight implants aren’t approved by FDA yet and not available in UAE or USA.
Phasing out of “Textured Implants”
In recent years, textured breast implants have been linked with a rare type of immune system disorder called as breast implant associated anaplastic large cell lymphoma (BIA-ALCL). This was first reported in 1997. So far around 800 cases have been reported with textured implants worldwide. Treatment in patients with BIA-ALCL is aimed at implant removal and total capsulectomy. Worldwide textured implants have been phased out and currently smooth implants are being used to prevent occurrences of BIA- ALCL.
Proper Implant size selection :
Implant size selection is mainly determined by the breast foot print, which is the width of the breast, also taking into consideration patients desires. Many times there is significant difference in the volume of right and left breast. We now have technologies to three dimensionally evaluate the breast volume and hence choose different size of breast implant to make the augmented breast look more symmetric.
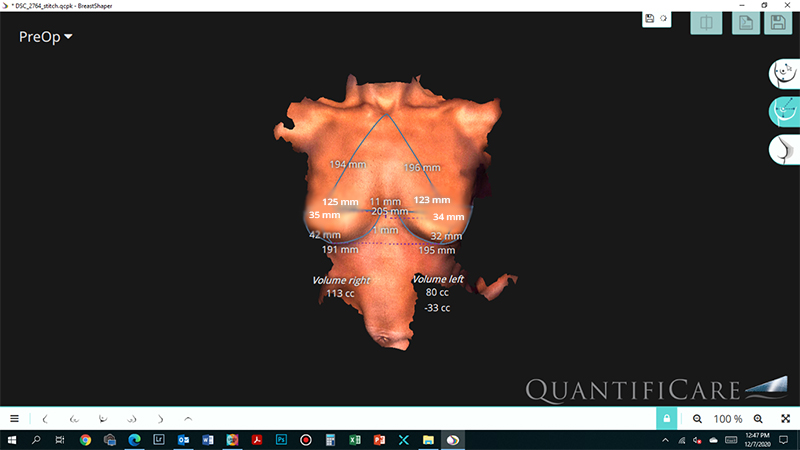
Fig. 2 : 3D analysis of breasts show left breast smaller than the right, hence a larger size of implant was used on the left side ( 335cc) and a smaller one on the right side(315cc) based upon the pre operative evaluation and patient agreement.
3D – analysis of the breast gives an objective quantification of breast volume and predictable outcome of the results.
Post Breast Implant screening :
The FDA recommends that people with silicone implants get regular screenings to detect silent ruptures.
For asymptomatic patients, the first ultrasound or magnetic resonance imaging (MRI) should be performed at 5-6 years postoperatively, then every 2-3 years thereafter. For symptomatic patients or patients with equivocal ultrasound results for rupture at any time postoperatively, an MRI is recommended, whether implants are for cosmetic augmentation or reconstruction. These recommendations do not replace other additional imaging that may be required depending on medical history or circumstances (i.e., screening mammography for breast cancer).
Additionally, FDA is also recommending ultrasound as an acceptable alternative to MRI for screening asymptomatic patients with breast implant in situ. These additional labeling recommendations were discussed at the March 2019 Panel Meeting.
Breast implant aren’t lifetime devices. FDA advices that they need to be changed after 10 years of surgery.
Breast Implant warranty:
Ask your Plastic Surgeon regarding the Implant warranty. Many implant manufacturers provide implant warranty against implant rupture and capsular contracture.
Silicone breast implant are not akin to silicone, so beware:
Lastly, silicone used for breast implants is different than injectable silicone. Injectable silicone is not FDA-approved for breast augmentation, breast reconstruction, or for any body contouring.
Always chose a board certified Plastic Surgeon to undergo breast augmentation or any cosmetic and other plastic surgery procedures to assure safety and predictable outcomes.
About myself: I am a Board Certified Plastic Surgeon with over 10 years of experience. Breast Surgeries remain among the most skill based procedure performed by Plastic Surgeons. I enjoy performing the surgery but more content to see when my patients step in our clinic with greater confidence and a whole new and improved version of themselves. You can reach me at [email protected] for any further enquiries on Breast Augmentation. I will be happy to answer them for you.